Buffer, pH control, acid-base balance, buffer solutions
By A Mystery Man Writer
Description
Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Ions are atoms or molecules that have lost or gained one or more electrons. An example of a common buffer is a solution of acetic acid (CH3COOH) and sodium
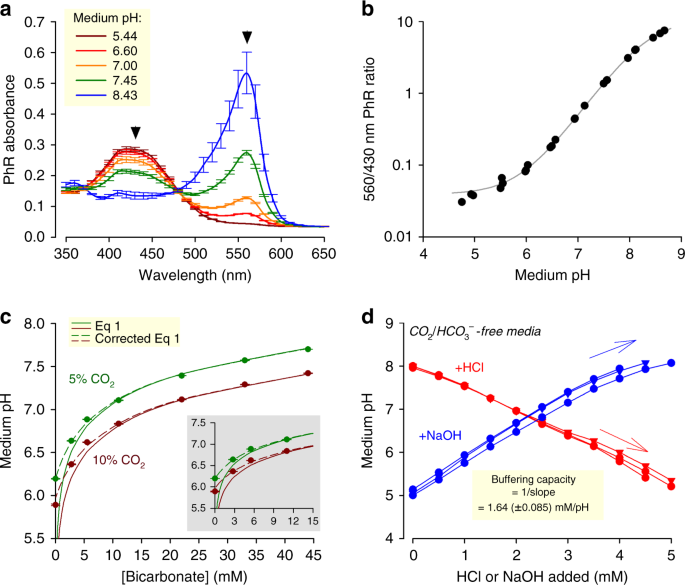
Evidence-based guidelines for controlling pH in mammalian live-cell culture systems

6.2 – Buffer Solutions – General Chemistry for Gee-Gees

Lecture 7 (acid base balance)
Buffer Solutions: Principle and Mechanism of their Action - PSIBERG
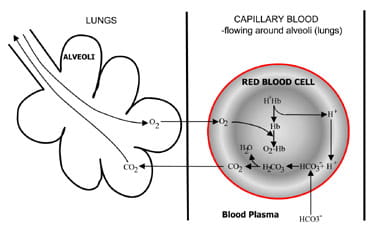
An introduction to acid-base balance in health and disease
27.4B: Chemical Buffer Systems - Medicine LibreTexts

Buffers 2 - Acid Base Equilibria - MCAT Content
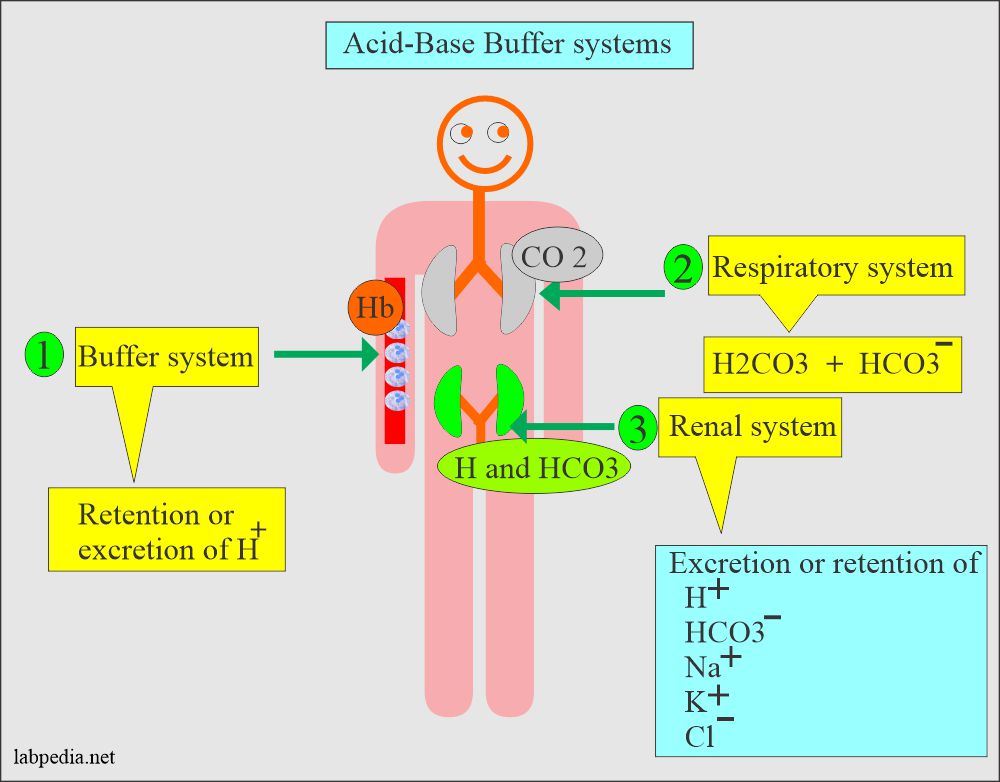
Acid-Base Balance:- Part 1 B - Acid-Base System

Blood pH BioNinja
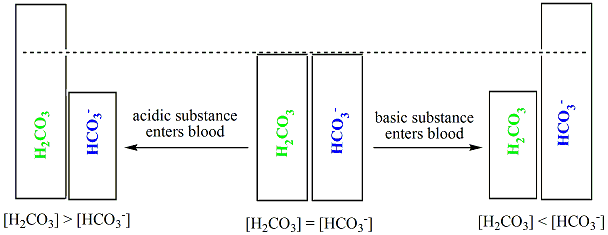
Chemistry of buffers and buffers in our blood (article)
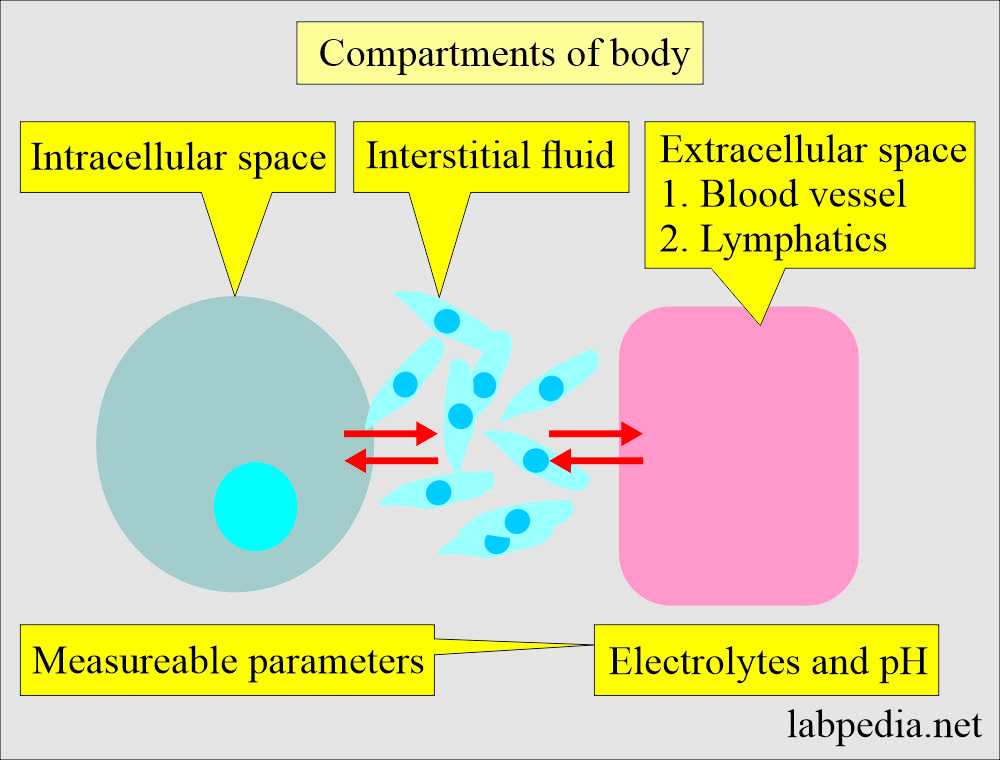
Acid-base Balance:- Part 1 A - Introduction to the Acid-Base Balance
from
per adult (price varies by group size)